My team at IBM’s research lab in the
Silicon Valley just discovered a new class of “self-healing” organogels that have
unique recyclable properties – they are the first class of chemically-crosslinked
gels that can be cooled to a solid, but then re-heated back to a liquid state. Imagine
filling a mold with this liquid material, cooling it and discovering there was
a mistake – with this material and its dynamic properties, we can start over until
we get the desired shape, and then cure it to a permanent, hardened object. We
describe how this process works in the paper, Melt-Processable
Dynamic-Covalent Poly(Hemiaminal) Organogels as Scaffolds for UV-Induced
Polymerization, published in the journal, Advanced Materials.
What is a gel?
What is a gel?
Gels are a peculiar family of materials. They exhibit properties between solids and liquids, and are composed of long polymer chains which link together and can trap some other smaller molecules. They are like a tiny (think nanoscale) fishing net that can retain liquids. The small molecules, which are trapped in the organogels that we developed, are monomers (the initial molecules which are used to prepare polymers). In our experiment, we also added some molecules called initiators in the gels, which under UV light transform the monomers into polymers. Thus, our gels have unique properties that allow them to behave at first like jello but after UV exposure become hard like plastic.
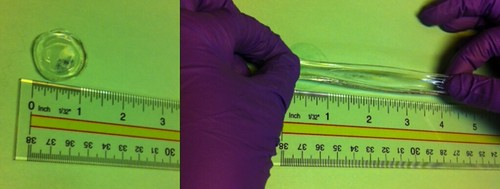 |
|
The
elasticity of our organogel on display
|
Think of our gels like caulk used to repair cracks in objects, but liquid enough to penetrate into small cracks, and solid enough not to leak. A subsequent UV-curing step would allow for the material filling the crack to solidify and for the object to recover properties close to its original shape and strength.
In the future, those gels could be used as a material for 3D-printing. If you think about today’s 3D-printing process, it requires layers of polymers stacked
on top of each other. This leads to imperfect shapes that can have weakly bonded interfaces between its layers. Our new “self-healing”
materials are not only fluid enough to be printed but also solid enough to hold
their shape. By precisely controlling the printing temperature, we could
ultimately get rid of the layers’ interface problem.
 |
|
IBM’s gel
(B), once heated, returns to its original state demonstrating
recyclable, remoldable properties. A typical gel (A) retains its form with heat. |
A lab legacy
This gel
discovery stems from work by IBM scientists Jim Hedrick and Jeannette Garcia
two years ago, when they discovered how to synthesize industrial
polymers. We applied this chemistry to our gels using computational
chemistry – co-author and IBMer Gavin Jones simulated the affinity our organogels’
crosslinks with the different monomers we used, and showed that they bound in
a similar way as the original molecules used by Jim and Jeannette. Our team’s
rheology expert, Nancy Zhang, also measured the gel’s ability to flow, and its strength.
Her data explained how to perceive the gel’s hardness and softness, and also
proved that the gel could be remolded multiple times before losing its strength.
Here’s
Nancy explaining the gel’s ability to be molded multiple times:


No comments:
Post a Comment